TRB Chemedica (UK) Ltd
TRB Chemedica (UK) Ltd is the official UK Distributor of OSTENIL, OSTENIL Plus, OSTENIL Mini, OSTENIL Tendon, VISCOSEAL, ArthroZheal PRF Surgical and MSK products, Clarius Ultrasound Scanners, and VISMED Eye Drops. TRB Chemedica (UK) provides proprietary, ethical, and research-based products for use in rheumatology, ophthalmology, and orthopaedics” in the UK and Ireland. In February of 2011 TRB Chemedica (UK) Ltd achieved ISO 9001 accreditation. This underlines the company's commitment to its customers and to its focus on quality. It also demonstrates that the company provides a high quality service throughout all its business operations.
Shop Ostenil
-
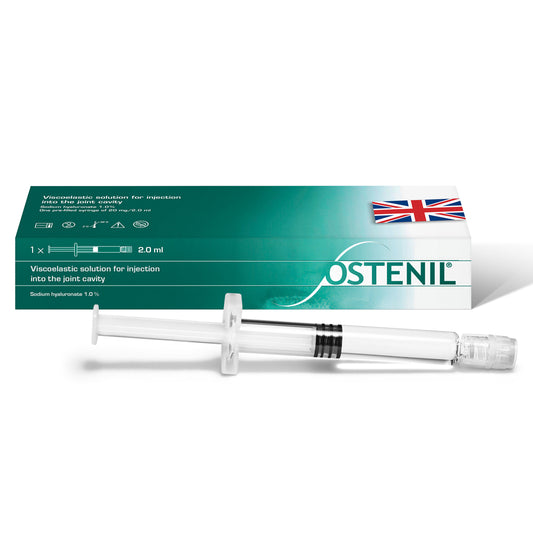
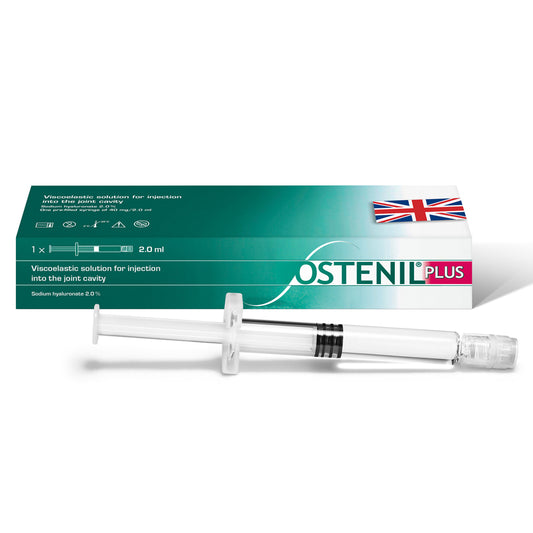
OSTENIL PLUS
Regular price £83.70 GBPRegular priceUnit price per -
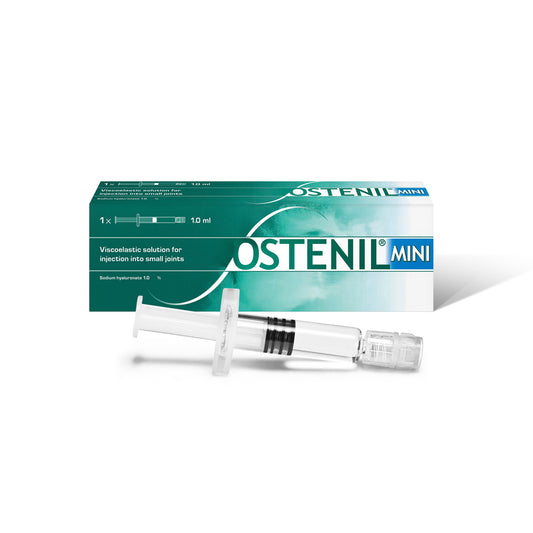
OSTENIL MINI
Regular price £25.00 GBPRegular priceUnit price per -
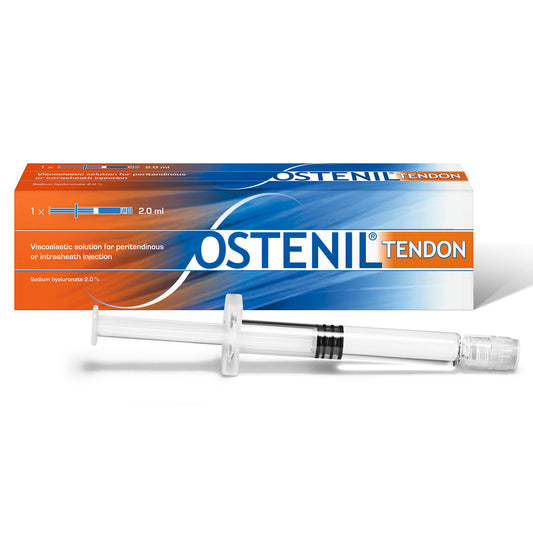
OSTENIL TENDON
Regular price £83.70 GBPRegular priceUnit price per
Shop Vismed
-
VISMED MULTI
Regular price £8.75 GBPRegular priceUnit price per -
VISMED GEL
Regular price £7.50 GBPRegular priceUnit price per -
VISMED GEL MULTI
Regular price £10.00 GBPRegular priceUnit price per